An Introduction to Snow Dynamics
There’s more to snow than just freezing some water.

Snow is one of the most important forms water can take. It can be devastating in some cases, and fun in others. Mountain snowpack is critical to water supplies, especially in the states of the Intermountain West. It disrupts travel, is the lifeblood of the ski industry, and is beautiful to behold.
Snow, of course, is associated with cold temperatures, and how cold those temperatures are has a tremendous influence on the duration and intensity of snow. Likewise, the ratio of moisture to snow is a function of temperature, density, and humidity.
The forecaster’s challenge
The dynamic nature of the atmosphere and the constantly changing conditions make forecasting snow accumulations uniquely challenging. According to a presentation by Greg DeVoir (link below) during a National Weather Service winter weather workshop in 2002, forecasters use a general rule of thumb: (intensity) x (duration)=snow amount.
Forecasters, using numerical models, are generally good a predicting duration of a particular snow event, but the intensity is more difficult. Factors such as terrain or orographic lift, convective stability, snow-to-water ratio (density), and precipitation phase all factor in along with surface temperatures. In addition, the size and shape of snowflakes are influenced by the intensity of lift, variations in moisture, and the vertical thermal profile and play an important role in resulting accumulations.
Most of us are familiar with the standard 10:1 snow-to-water ratio. According to DeVoir, however, the 10:1 ratio is accurate only 25.8 percent of the time. Observational studies resulted in snow ratios of fresh snow varying from 3:1 to as much as 100:1. Evan Kuchera, a US Air Force meteorologist, developed the Kuchera ratio which attempted to refine the 10:1 ratio to improve its accuracy, but whether Kuchera’s method is an improvement is subject to debate, though many weather sites include maps based on his methodology.

Let’s start with temperature
Temperatures consistently above freezing don’t produce a lot of snow. That applies not only to ground level but, more importantly, to the air above us. Water vapor suspended in the atmosphere in the form of clouds is at or very near total saturation. A given volume of atmosphere is essentially holding all of the moisture it can at that temperature.
Much of the water vapor is supercooled, i.e. it is below freezing yet still in liquid form. As soon as these supercooled droplets moving around within the cloud come into contact with a foreign particle, whether ice, dust, ash, or another atmospheric pollutant, they immediately freeze and adhere to the particle in a process called accretion and riming. As the frozen particles continue to move, they contact more droplets and gain more weight and size due to accretion until they are heavy enough for gravity to pull them downward. It may seem obvious, but in the atmosphere, no ice means no snow.
The most common form that most of us associate with snowfall is dendritic snowflakes. These are the intricate, somewhat star-shaped snowflakes that come to mind when we talk about snow. Snow, however, can take many forms depending on the temperature and saturation of the atmosphere at the level at which they are forming.
The size and shape of the snow at this point are temperature-dependent, as seen in Figure 1, below:
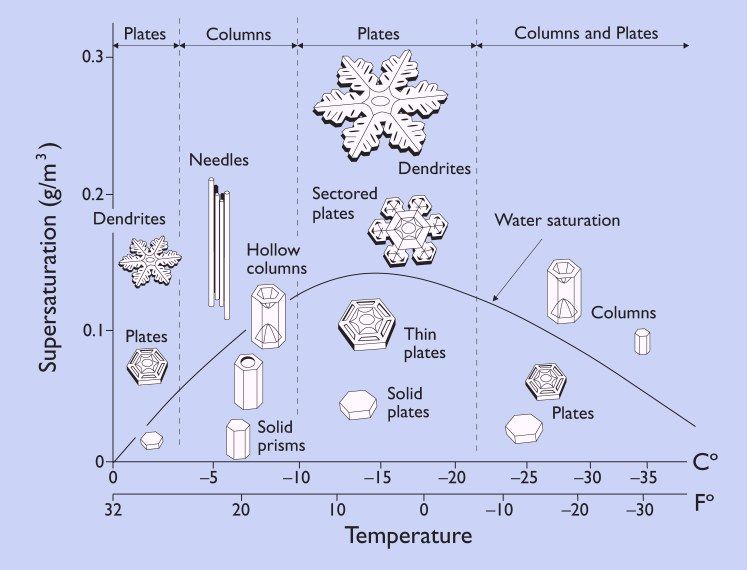
The graphic above is instructional when it comes to the various forms of frozen precipitation we may encounter. For instance, at warmer temperatures, around -5°C, a few dendrites may be present but the crystals more typically tend to form six-sided plates. Between -5°C and -10°C, needles, hollow columns, capped columns, and solid prisms, again six-sided, are more common. At temperatures below -20°C, again, snow tends to take the forms of columns, prisms, and plates.
The Dendritic Growth Zone
The area between -10°C and -18°C is the sweet spot for snow. This temperature range is known as the Dendritic Growth Zone (DGZ) and is most favorable for the formation of classic dendritic snowflakes, sectored plates, thin plates, and solid plates. It is also the peak temperature range for the water saturation curve, as shown in Fig. 1.
Figure 2 below is a sounding from the area of southwest Colorado's San Juan Mountains where a snowstorm was forecast. The area between -10°C and -18°C marked with yellow is the dendritic growth zone and the altitude and depth of the zone are represented to the right of the temperature line. Note also that the temperature profile (red) and the dew point profile (green) overlay each other to the 200 Mb line, indicating a very deep layer of saturated atmosphere.
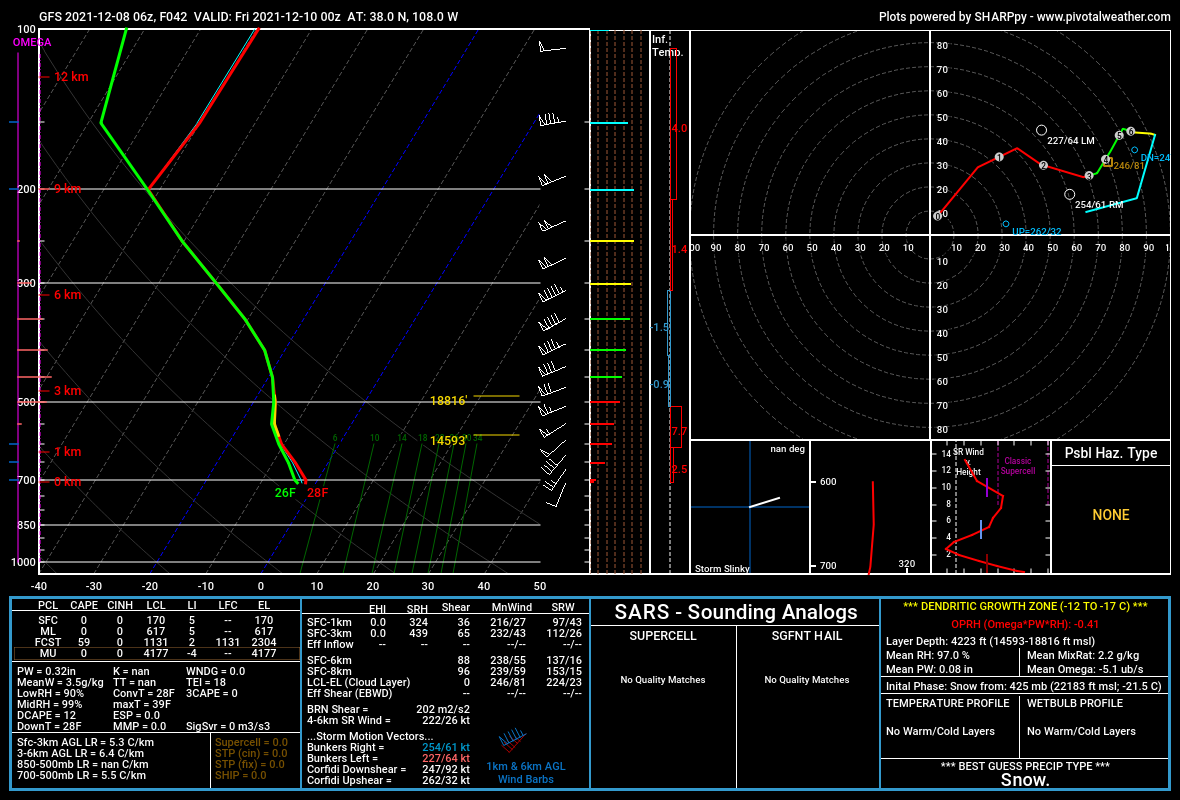
The bottom of the dendritic growth zone is calculated as 14,593 feet elevation and the top at 18,816 feet, giving a depth of 4,223 feet, prime for dendritic snowflake formation. Additional information on the DGZ is found in the lower right corner of the sounding. This particular storm dropped several feet of snow in southwestern Colorado.
Why are snowflakes hexagonal?
Snowflakes tend to form hexagonal patterns, whether they take the form of dendrites, columns, or plates. The reason for this lies in the molecular structure of water, i.e. the relationship between the hydrogen and oxygen atoms and the bond between the molecules of H2O.
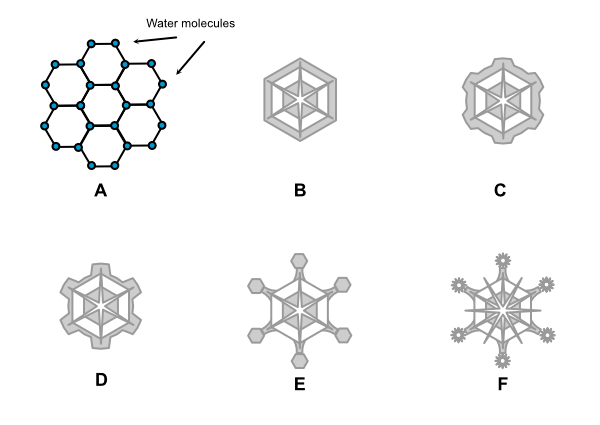
Looking at A in Figure 3 above, water molecules form a natural hexagon at the molecular level. This is the basis for ice crystal structure. In B, the hexagonal platelet forms in the atmosphere. Because the corners stick out farther into the humid air, ice crystals accrete on these corners at a faster rate. The snowflake begins to take the form shown in C. As accretion continues, the snowflake takes on the dendrite form (D). As the arms continue to grow, plates may form on the ends and the process begins again (E), with the points of the plates accreting more ice and the dendrite becoming ever more intricate (F) as it moves through varying temperatures and moisture levels before falling to the ground.
This is, of course, a highly simplified version; myriad influences determine the final shape, size, and growth rate, which is why there are no two identical snowflakes. The chaotic dynamics of a fluid atmosphere that is constantly in motion ensure never-ending variations.
Studying the shapes, sizes, and formation dynamics of snow is not only fascinating by itself but is also extremely important in understanding and forecasting snowfall. A basic understanding of the physics of snow will help you be a better observer.

